Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concept of intermolecular forces, the attractive forces between molecules.
ii. Describe the three main types of intermolecular forces: dipole-dipole interactions, hydrogen bonding, and London dispersion forces.
iii. Compare and contrast the strength and nature of intermolecular forces in solids, liquids, and gases.
iv. Explain how intermolecular forces influence the physical properties of matter, such as melting point, boiling point, and viscosity.
v. Recognize the interplay between intermolecular forces and the changes in matter between its solid, liquid, and gaseous states.
Introduction
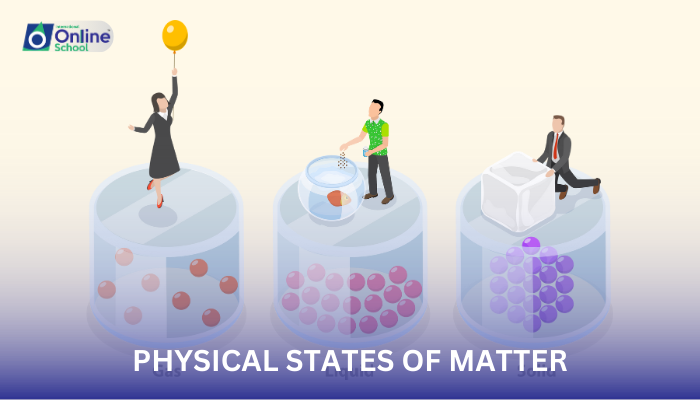
The world around us is a captivating tapestry of matter, existing in three distinct states: solid, liquid, and gas. Each state exhibits unique properties, and at the heart of these differences lie intermolecular forces, the invisible yet powerful interactions between molecules.
i. Intermolecular Forces: The Invisible Bonds
Intermolecular forces, the attractive forces between molecules, are responsible for the cohesion of matter, holding molecules together. These forces vary in strength and nature, influencing the physical properties of matter, including its state of existence.
ii. Dipole-Dipole Interactions: A Dance of Polar Molecules
Dipole-dipole interactions arise between molecules with permanent dipoles, resulting from the unequal distribution of electrons. These interactions involve the attraction between the positive end of one molecule and the negative end of another.
iii. Hydrogen Bonding: A Special Kind of Dipole-Dipole Interaction
Hydrogen bonding, a particularly strong type of dipole-dipole interaction, occurs between molecules containing a hydrogen atom bonded to a small, highly electronegative atom, such as nitrogen, oxygen, or fluorine. The strength of hydrogen bonding is due to the high electronegativity of the acceptor atom and the partial positive charge on the hydrogen atom.
iv. London Dispersion Forces: A Universal Attraction
London dispersion forces, the weakest type of intermolecular force, exist between all molecules, regardless of their polarity. These forces arise from temporary fluctuations in electron distribution, creating instantaneous dipoles that attract neighboring molecules.
v. Comparing Intermolecular Forces in Solids, Liquids, and Gases
The strength and nature of intermolecular forces differ significantly between the three states of matter:
Solids: In solids, intermolecular forces are strongest, holding molecules in a fixed arrangement. This rigidity results in the well-defined shape and resistance to deformation characteristic of solids.
Liquids: In liquids, intermolecular forces are weaker than in solids, allowing molecules to move past each other but remain close together. This fluidity gives liquids their ability to flow and take the shape of their container.
Gases: In gases, intermolecular forces are extremely weak, allowing molecules to move freely and occupy a large volume. This freedom of movement leads to the expansion and diffusion properties observed in gases.
vi. Intermolecular Forces and Physical Properties
Intermolecular forces play a crucial role in determining the physical properties of matter:
Melting Point: The stronger the intermolecular forces, the higher the melting point, the temperature at which a solid transitions to a liquid.
Boiling Point: The stronger the intermolecular forces, the higher the boiling point, the temperature at which a liquid transitions to a gas.
Viscosity: The stronger the intermolecular forces, the higher the viscosity, the resistance of a liquid to flow.
vii. Changes of State and Intermolecular Forces
The transition between the solid, liquid, and gaseous states is governed by the interplay between intermolecular forces and the kinetic energy of molecules. As energy increases, intermolecular forces are overcome, allowing molecules to move more freely and the state of matter to change.
Intermolecular forces, the invisible forces between molecules, play a profound role in shaping the physical world around us. By understanding the nature and strength of these forces, we gain valuable insights into the properties of solids, liquids, and gases, their behavior under various conditions, and the fascinating transitions between these states of matter.